Alnylam Pharmaceuticals


ABOUT ALNYLAM
Our Science Is Changing the Way Medicine Treats DiseaseTM
Alnylam has led the translation of RNAi (RNA interference) from Nobel Prize-winning discovery into an innovative, entirely new class of medicines. Founded in 2002 by a team of distinguished life sciences leaders, Alnylam’s vision is to harness the potential of RNAi therapeutics to transform the lives of people living with diseases for which there are limited or inadequate treatment options. Our pioneering work has delivered the world’s first and only approved RNAi therapeutics—ONPATTRO® (patisiran) in 2018, GIVLAARI® (givosiran) in 2019, OXLUMO® (lumasiran) in 2020, and AMVUTTRA® (vutrisiran) in 2022. We are advancing a deep pipeline of innovative RNAi-based medicines in four therapeutic areas: genetic medicines, cardio-metabolic diseases, infectious diseases, and central nervous system (CNS) and ocular diseases.

What’s in a Name?
AL-NY-LAM. Our name may not be the easiest to pronounce, but once you learn it, you’ll never forget it. Alnylam is derived from “Alnilam,” the bright center star in the constellation Orion’s belt, which has been used by navigators for thousands of years and symbolizes our passion for discovery.
RNAi Therapeutics—An Innovative New Class of Medicines
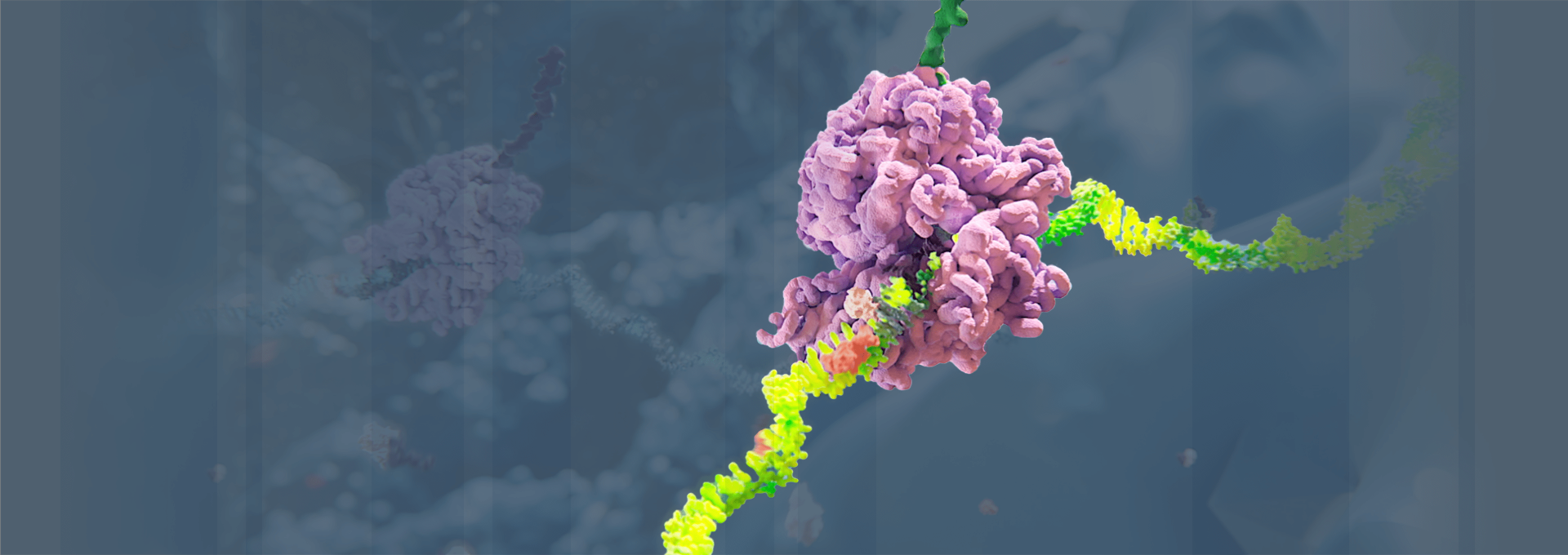
Medicines based on RNAi work by “silencing” or disabling the production (“expression”) of the genes that cause specific diseases. In doing so, RNAi therapeutics work “upstream” of most other classes of medicines, such as small molecules and monoclonal antibodies, by targeting the “root” genetic cause of a disease rather than its symptoms. To learn more about our science, click here.
RNAi is the core discovery that forms the therapies Alnylam is developing. It is recognized as a major scientific breakthrough—but how does it work, exactly?
Vision, Mission, and Values
Our Vision - Harnessing a revolution in biology for human health®.
Our Mission - Build a top-tier, global, independent biopharmaceutical company founded on RNAi.
Our Core Values - Commitment to People, Innovation & Discovery, Sense of Urgency, Open Culture, and Passion for Excellence.
CAREERS AT ALNYLAM
Join Our Team and Grow With Us
At Alnylam, we are driven by our mission to translate the Nobel Prize-winning discovery of RNA interference (RNAi) into an innovative, new class of medicines. We’re motivated in this endeavor by the bravery and perseverance of people with unmet medical needs who live with rare and genetic diseases. They are the focus of our efforts, and we exist as a company because of them. That knowledge and commitment to patients is present in everything we do, every day.

At The Forefront of What’s Next
For Patients
Our goal, which is well underway, is to become a world-class biopharmaceutical company that can independently develop and deliver innovative medicines based on RNAi to patients worldwide. We have a deep pipeline of early to late stage investigational therapies, and 2018 marked the approval of our first medicine—the world’s first RNAi therapeutic. Even though we’ve been around since 2002, we’re just getting started!
For Employees
We are growing quickly and hiring across North America, Europe, Asia, and Latin America for a wide variety of roles. We’re seeking smart, passionate, “change the world” kind of people who are ready to say, “challenge accepted.” Our 1,500 (and counting!) employees are building a diverse, equitable, inclusive, and high-performing global organization that celebrates team achievement and recognizes individual contributions.
We’re at the forefront of what’s next for patients, and perhaps your career also.
Search among Alnylam Pharmaceuticals jobs
| Jobs: 11 - 20 of 93 |

Executive Director, Head of Business Development Transactions
Cambridge, Massachusetts
Overview For the Executive Director, Head of BD Transactions, we are seeking an experienced business development (BD) leader with strong business acumen with deep business development experience in the biotechnology and biopharmaceutical in...
11h
| Job Type | Full Time |

Director, Global Trade Compliance and Strategy
Cambridge, Massachusetts
Overview The Director, Head of Global Trade Compliance and Strategy is a key supply chain leader responsible for designing, implementing, and overseeing the company's global trade compliance program while shaping trade-related strategy to s...
1d
| Job Type | Full Time |

Associate Scientist II, Conjugation Chemistry
Cambridge, Massachusetts
Overview We are seeking an associate scientist-II to join our protein sciences team to contribute to our protein production and conjugation efforts. The candidate must be independent, goal oriented, technically competent, and able to effici...
1d
| Job Type | Full Time |

Associate Scientist II, Protein Sciences
Cambridge, Massachusetts
Overview We are seeking an associate scientist-II to join our protein sciences team to contribute to our protein production and conjugation efforts. The candidate must be independent, goal oriented, technically competent, and able to effici...
1d
| Job Type | Full Time |

Scientist, Research (Targeted Delivery)
Cambridge, Massachusetts
Overview The Research Department at Alnylam is seeking a Scientist to join a growing team responsible for advancing RNAi therapeutics into the future, by opening up targeted delivery to all tissues. The successful candidate will have a work...
1d
| Job Type | Full Time |

The Associate Director, Contract Manufacturing Pack and Label
Cambridge, Massachusetts
The Associate Director, Contract Manufacturing Pack and Label is a standalone role responsible for the strategic and operational management of contract manufacturers (CMOs) supporting commercial packaging and labeling operations within a de...
1d
| Job Type | Full Time |

Associate Legal Director, US Commercial
Cambridge, Massachusetts
Associate Director, Product Counsel Role Summary The Associate Director, Product Counsel will hold a pivotal position within the Commercial Legal Team, partnering with the US TTR marketing and sales teams to drive the success of the TTR fra...
1d
| Job Type | Full Time |

Associate Director, Federal Government Relations (Washington DC Location)
Cambridge, Massachusetts
Overview The Associate Director, Federal Government Relations, will advance Alnylam's public policy priorities at the federal level by leveraging political insights, building and maintaining relationships with key policymakers, representing...
1d
| Job Type | Full Time |

Senior Manager, Program Management
Cambridge, Massachusetts
Overview At Alnylam, clinical-stage development programs are managed by a Global Program Leadership Team, led by a Program Leader and a Program Manager, responsible for harnessing cross-functional expertise to define the best scientific, cl...
1d
| Job Type | Full Time |

Cambridge, Massachusetts
Overview The Drug Innovation group at Alnylam is seeking a passionate, creative post-doctoral scientist to join our highly interdisciplinary team focused on the advancement of our RNAi Platform and Lead Development efforts through novel app...
1d
| Job Type | Full Time |

