Alnylam Pharmaceuticals


ABOUT ALNYLAM
Our Science Is Changing the Way Medicine Treats DiseaseTM
Alnylam has led the translation of RNAi (RNA interference) from Nobel Prize-winning discovery into an innovative, entirely new class of medicines. Founded in 2002 by a team of distinguished life sciences leaders, Alnylam’s vision is to harness the potential of RNAi therapeutics to transform the lives of people living with diseases for which there are limited or inadequate treatment options. Our pioneering work has delivered the world’s first and only approved RNAi therapeutics—ONPATTRO® (patisiran) in 2018, GIVLAARI® (givosiran) in 2019, OXLUMO® (lumasiran) in 2020, and AMVUTTRA® (vutrisiran) in 2022. We are advancing a deep pipeline of innovative RNAi-based medicines in four therapeutic areas: genetic medicines, cardio-metabolic diseases, infectious diseases, and central nervous system (CNS) and ocular diseases.

What’s in a Name?
AL-NY-LAM. Our name may not be the easiest to pronounce, but once you learn it, you’ll never forget it. Alnylam is derived from “Alnilam,” the bright center star in the constellation Orion’s belt, which has been used by navigators for thousands of years and symbolizes our passion for discovery.
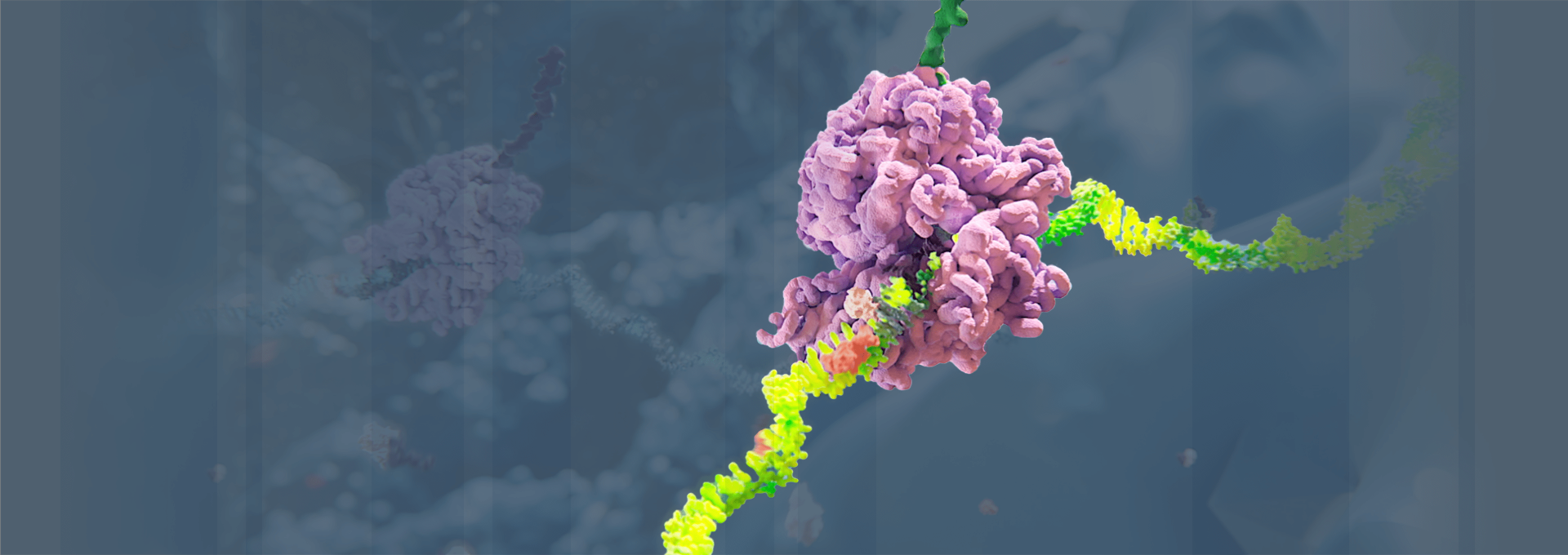
RNAi Therapeutics—An Innovative New Class of Medicines
Medicines based on RNAi work by “silencing” or disabling the production (“expression”) of the genes that cause specific diseases. In doing so, RNAi therapeutics work “upstream” of most other classes of medicines, such as small molecules and monoclonal antibodies, by targeting the “root” genetic cause of a disease rather than its symptoms. To learn more about our science, click here.
RNAi is the core discovery that forms the therapies Alnylam is developing. It is recognized as a major scientific breakthrough—but how does it work, exactly?
Vision, Mission, and Values
Our Vision - Harnessing a revolution in biology for human health®.
Our Mission - Build a top-tier, global, independent biopharmaceutical company founded on RNAi.
Our Core Values - Commitment to People, Innovation & Discovery, Sense of Urgency, Open Culture, and Passion for Excellence.
CAREERS AT ALNYLAM
Join Our Team and Grow With Us
At Alnylam, we are driven by our mission to translate the Nobel Prize-winning discovery of RNA interference (RNAi) into an innovative, new class of medicines. We’re motivated in this endeavor by the bravery and perseverance of people with unmet medical needs who live with rare and genetic diseases. They are the focus of our efforts, and we exist as a company because of them. That knowledge and commitment to patients is present in everything we do, every day.

At The Forefront of What’s Next
For Patients
Our goal, which is well underway, is to become a world-class biopharmaceutical company that can independently develop and deliver innovative medicines based on RNAi to patients worldwide. We have a deep pipeline of early to late stage investigational therapies, and 2018 marked the approval of our first medicine—the world’s first RNAi therapeutic. Even though we’ve been around since 2002, we’re just getting started!
For Employees
We are growing quickly and hiring across North America, Europe, Asia, and Latin America for a wide variety of roles. We’re seeking smart, passionate, “change the world” kind of people who are ready to say, “challenge accepted.” Our 1,500 (and counting!) employees are building a diverse, equitable, inclusive, and high-performing global organization that celebrates team achievement and recognizes individual contributions.
We’re at the forefront of what’s next for patients, and perhaps your career also.
Search among Alnylam Pharmaceuticals jobs
| Jobs: 1 - 10 of 93 |

Senior Manager, Standards, Process & Training
Cambridge, Massachusetts
Overview Reporting to the Associate Director of Standards, Process & Training - Clinical Development, the Sr Manager, GCP Process Managementserves as a process excellence Subject Matter Expert (SME) on process improvement, management and ef...
5h
| Job Type | Full Time |

Cambridge, Massachusetts
Overview The Manager, Clinical Operations is responsible for the execution of study-level activities including but not limited to creating and updating trial-specific documents, vendor oversight & delivery, compound training and country and...
5h
| Job Type | Full Time |

Patient Education Liaison - Rare Disease (South-Central Region)
Cambridge, Massachusetts
Overview Reporting to the Director, US Patient Services, the Patient Education Liaison (PEL) is a critical component of the product brand commercial strategy and works in close coordination with Field Sales, Field Reimbursement, Patient Ser...
10h
| Job Type | Full Time |

Executive Director, Head of Search & Evaluation, Business Development
Cambridge, Massachusetts
Overview For the Executive Director, Head of Search & Evaluation role, we are seeking an experienced business development (BD) leader with deep scientific expertise and passion for innovation to source and evaluate external innovation and b...
11h
| Job Type | Full Time |

Associate Director, Clinical Pharmacology
Cambridge, Massachusetts
Overview The Associate Director in Clinical Pharmacology position will support a rapidly expanding clinical portfolio of promising RNAi therapeutics. The candidate in this position should be well versed in clinical pharmacology, PK and PD w...
11h
| Job Type | Full Time |

Business Account Executive, Rare Disease - Minneapolis, MN
Cambridge, Massachusetts
Overview We are looking for an experienced and talented Business Account Executive (BAE) to commercially represent GIVLAARI and OXLUMO in the United States and have a significant impact on the lives of AHP patients. AHP's represent a sub-gr...
11h
| Job Type | Full Time |

Specialist, Materials Management
Cambridge, Massachusetts
Overview As a Specialist, Materials Management at Alnylam, you will play a crucial role in ensuring the continuos, uninterrupted flow of raw materials to support both internal and external manufacturing operations. Reporting to the Senior M...
11h
| Job Type | Full Time |

Executive Director, Pharmacometrics
Cambridge, Massachusetts
Overview Seeking an experienced Head of Pharmacometrics to expand Clinical Pharmacology and Pharmacometrics capabilities within Alnylam. The Executive Director, Pharmacometrics is a on-site, leadership role focused on providing strategic di...
11h
| Job Type | Full Time |

Cambridge, Massachusetts
Overview The Director, Feasibility Center of Excellence (CoE) will be responsible for leading and managing feasibility strategy and execution across multiple clinical development programs. Reporting to the Sr. Director of the Feasibility Ce...
11h
| Job Type | Full Time |

Senior Manager, Clinical Trial Disclosure and Transparency
Cambridge, Massachusetts
The Senior Manager, Clinical Trial Disclosure and Transparency is a key member of the Clinical Trial Disclosure and Transparency (CTDT) team working within the Alnylam Medical Writing Expertise Area. The Senior Manager, CTDT is responsible ...
11h
| Job Type | Full Time |

